Dapagliflozin is approved for treating paediatric type 2 diabetes
- Dr. Geetika Gupta

- Sep 10, 2025
- 6 min read

Type 2 diabetes mellitus (T2D) is a progressive metabolic disease that affects members of all ages but is more common in people over 45 years (1). Type 2 diabetes has recently become more common among children, adolescents, and young adults around the world. In 2021, about 41,600 new cases of youth-onset diabetes were reported worldwide (2). The prevalence of youth-onset T2D among children aged 10–19 is 0.67 per 1000, a 95.3% increase since 2001. Additionally, 1 in 5 adolescents aged 12 to 18 years has prediabetes as per the National Health and Nutrition Examination Survey (NHANES) data from 2005 to 2016. Factors like obesity, physical inactivity, and consumption of energy-dense diets contribute to this upward trend (3).
Youth-onset type 2 diabetes progresses more quickly, with greater rates of complications and 1.5 times higher short-term mortality compared to type 1 diabetes (4). Despite the increasing prevalence of type 2 diabetes and its consequences in children and adolescents, oral treatment options remain limited (3). Many young patients struggle with poor blood sugar control leading to severe complications in the prime of their lives (5).
Complications and Treatment Challenges
Youth-onset type 2 diabetes leads to early and multiple complications within 15 years of diagnosis. The TODAY and TODAY2 studies found that 60% of youth with diabetes developed a minimum of one complication, and nearly 30% had two or more complications. Despite various treatments, all groups experienced significant declines in blood glucose control, with high rates of hypertension (67%), dyslipidaemia (52%), kidney disease (55%), nerve disease (32%), and eye disease (51%). Significant cardiovascular events occurred even at a young age (5,6).
Metformin and GLP-1 receptor agonists are the only FDA-approved oral medications for children. Most anti-diabetic drugs have only been tested for safety and efficacy in adults, and insulin is used less often due to specific requirements and the risk of hypoglycaemia (4).
Youth-onset diabetes is more aggressive and harder to control than adult-onset diabetes. It needs to be treated intensely from diagnosis, utilizing comprehensive strategies to avoid, delay, and aggressively treat complications (6).
Farxiga (dapagliflozin) for the treatment of paediatric diabetes
Farxiga (dapagliflozin) is a first-in-class, oral, once-daily sodium-glucose cotransporter 2 (SGLT2) inhibitor. It is approved in 126 countries as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes mellitus (7). It works by preventing glucose reabsorption in the kidneys, leading to increased glucose excretion and reduced plasma glucose levels. In addition, Dapagliflozin has also been found to be both cardioprotective and renoprotective. Dapagliflozin reduces sodium reabsorption in the renal tubules, decreasing blood volume and pressure to benefit heart failure, while also reducing hyperfiltration to protect kidney function in chronic kidney disease. Farxiga (dapagliflozin) is approved for the treatment of heart failure across the entire ventricular ejection fraction range (HFrEF and HFpEF), as well as CKD in adults (8).
Recently, Farxiga (dapagliflozin) has also been approved by the US Food and Drug Administration (FDA) for improving glycemic control in pediatric patients aged 10 years and older with type 2 diabetes, based on positive results from the paediatric T2NOW Phase III trial (9,10).
Clinical Evidence - T2NOW study
The T2NOW study is a randomized, double-blind, placebo-controlled Phase III trial, conducted to evaluate the efficacy and safety of Farxiga (dapagliflozin) as an add-on treatment in children and adolescents with type 2 diabetes (T2D) receiving metformin, insulin, or both.
Participants were assigned to receive either dapagliflozin, saxagliptin, or a placebo. Those on an active medication were further randomized to either maintain their current dose or increase it to a higher dose. The primary endpoint was the change in A1C levels after 26 weeks compared to placebo for dapagliflozin (5 or 10 mg) and saxagliptin (2.5 or 5 mg) as the active treatments. Secondary endpoints included changes in fasting plasma glucose and the proportion of patients with baseline A1C ≥7% achieving A1C <7% (53 mmol/mol) after 26 weeks (10).
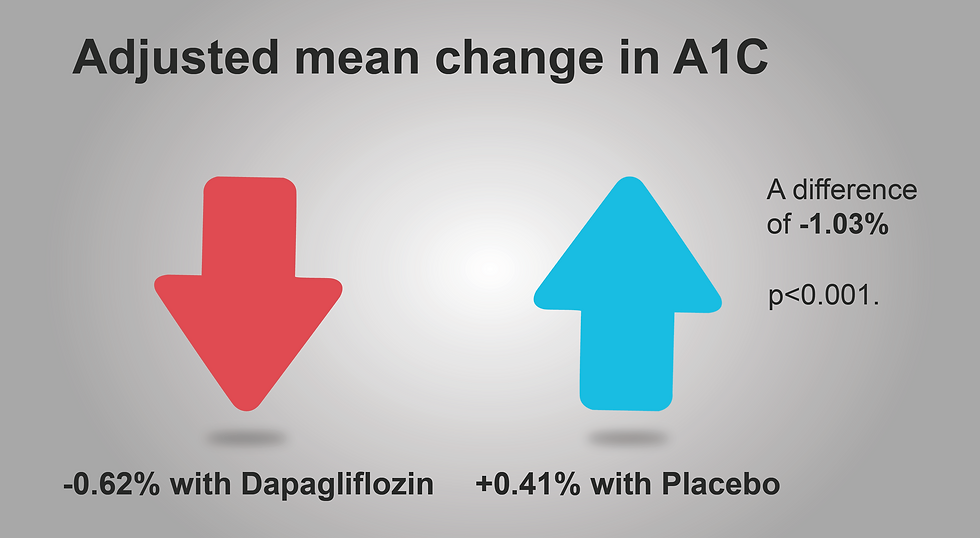
Data from the T2NOW trial demonstrated a significant reduction in A1C with Farxiga (dapagliflozin) compared to those receiving placebo. The average A1C level reduced by 0.62% for those taking Farxiga (dapagliflozin), while it increased by 0.41% for those on placebo (p<0.001) (10).
At week 26, both primary endpoint and the secondary endpoints were statistically significant when compared to placebo. These findings demonstrated that Farxiga (dapagliflozin) can provide clinically substantial improvements in blood sugar control for children and adolescents with T2D. The safety results for this cohort were consistent with those seen in adults with type 2 diabetes (10).
Conclusion
Type 2 diabetes is becoming more prevalent in pediatric population, with rapid disease progression and a higher risk of complications. Oral treatment options for this population are still limited. Many young patients struggle with poor blood sugar management and treatment adherence, resulting in serious consequences. Youth-onset diabetes is more aggressive and should be handled aggressively from the start, including comprehensive measures to avoid, postpone, and treat complications. FDA approval for Farxiga (dapagliflozin) represents a breakthrough solution for the management of pediatric diabetes. Results from the T2NOW trial have shown Dapagliflozin (Farxiga) shows significant glycemic improvements in pediatric T2D with safety outcomes aligning with its established profile in adults. Farxiga has the potential to alter practice guidelines for diabetes and other pediatric illnesses.
Indications
FARXIGA (Dapagliflozin) is an SGLT2 inhibitor indicated:
as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (9)
along with diet and exercise to improve blood sugar (glucose) control in adults and children who are 10 years of age and older with type 2 diabetes (7).
to reduce the risk of hospitalization for heart failure in adults with type 2 diabetes mellitus and either established cardiovascular (CV) disease or multiple CV risk factors (9).
to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visit in adults with heart failure (9).
to reduce the risk of sustained eGFR decline, end-stage kidney disease, cardiovascular death, and hospitalization for heart failure in adults with chronic kidney disease at risk of progression (9).
Dosage and Administration
FARXIGA (Dapagliflozin) is available as an oral, once-daily 5mg or 10mg tablet.
In paediatric and adult patients with Type 2 DM: Initial: 5 mg once daily; May be increased to 10 mg once daily in patients tolerating 5 mg/day who require additional glycemic control (11).
In adult patients with CKD at risk of progression: 10 mg once daily (11).
In adult patients with heart failure: 10 mg once daily (11).
In adult patients with T2D and either multiple CV risk factors or eCVD: 10 mg once daily (11).
About the Author
Dr Geetika Gupta is a quirky wordsmith with a toothy grin. She has done her Bachelor of Dental surgery degree from Rajiv Gandhi University of Health sciences and is a gold medalist. Oral cancer, bone pathologies and implantology are some of her favorite topics. When she’s not busy filling cavities and perfecting smiles, she makes time to write articles on various healthcare topics.
References
Goyal R, Singhal M, Jialal I. Type 2 Diabetes. [Updated 2023 Jun 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
Wu H, et al. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. 2022 Mar;185:109785.
Rodriquez IM, O'Sullivan KL. Youth-Onset Type 2 Diabetes: Burden of Complications and Socioeconomic Cost. Curr Diab Rep. 2023 May;23(5):59-67.
George MM, Copeland KC. Current treatment options for type 2 diabetes mellitus in youth: today's realities and lessons from the TODAY study. Curr Diab Rep. 2013 Feb;13(1):72-80.
Serious complications from youth-onset type 2 diabetes arise by young adulthood. Available at: https://www.nih.gov/news-events/news-releases/serious-complications-youth-onset-type-2-diabetes-arise-young-adulthood Accessed on 19th June 2024.
TODAY Study Group; Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021 Jul 29;385(5):416-426.
Farxiga approved in the USfor the treatment of paediatric type-2 diabetes. Available at: https://www.astrazeneca.com/media-centre/press-releases/2024/farxiga-approved-in-the-us-for-the-treatment-of-paediatric-type-2-diabetes.html#! Accessed on 20 June 2024.
Grube PM, Beckett RD. Clinical studies of dapagliflozin in pediatric patients: a rapid review. Ann Pediatr Endocrinol Metab. 2022 Dec;27(4):265-272.
Farxiga (dapagliflozin) US prescribing information; 2024. Available at: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/0be9cb1b-3b33-41c7-bfc2-04c9f718e442/0be9cb1b-3b33-41c7-bfc2-04c9f718e442_pi_med_guide_rendition__c.pdfAccessed on 20 June 2024.
Shehadeh N, et al. Dapagliflozin or Saxagliptin in Pediatric Type 2 Diabetes. NEJM Evid. 2023 Dec;2(12):EVIDoa2300210.
Farxiga Dosing. Available at: https://www.farxiga-hcp.com/dosingAccessed on 20 June 2024.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@rxnews.in







Comments