Role of Levonadifloxacin against MDR infections
- Dr. Ujwala Kemkar

- Sep 9, 2025
- 5 min read
Updated: Sep 10, 2025
Antimicrobial resistance (AMR) has emerged as a major global health threat. The World Health Organization (WHO) has recognized AMR as a critical concern, projecting up to 10 million annual deaths due to drug-resistant infections by 2025 (1). Methicillin-resistant Staphylococcus aureus (MRSA), one of the known "superbugs," is a significant cause of antimicrobial-resistant infections worldwide, leading to high mortality rates, treatment cost, and longer hospital stay (1).
MRSA prevalence ranges from 13% to 74% globally, with India reporting around 37%. MRSA strains resist most β-lactam antibiotics via the staphylococcal cassette chromosome (SCCmec), with different types prevalent in hospital- and community-acquired MRSA. In India, the ST772-MRSA-V clone, known for its toxin production, leads to resistance against various antibiotics (2).
Global MRSA mortality rates range from 15% to 60%, and Indian studies report a 27% fatality rate in MRSA bacteraemia (2). Early detection and empiric treatment are crucial in effectively managing MRSA infections (3).
Limitations of existing antibiotics
Current treatment options for MRSA in India include vancomycin, teicoplanin, linezolid, daptomycin, and tigecycline. The table below summarizes the common limitations of currently available treatment (3).
Table 1: Common limitations of currently available antibiotics (3)

Unmet medical need in treating MRSA infections
The rising mortality and morbidity from MRSA infections persist despite the availability of multiple anti-MRSA antibiotics. This highlights the unmet need for effective management of various MRSA infections, particularly in patients with diverse comorbidities, which could pose a therapeutic challenge to older MRSA drugs (4). Key limitations of these older anti-MRSA antibiotics, such as glycopeptides, linezolid, daptomycin, and tigecycline, include suboptimal pharmacokinetics/pharmacodynamics (PK/PD), bacteriostatic or slow bactericidal action, safety concerns in critically ill patients, and the absence of an oral option (4).
Many of these older antibiotics also lack the broad-spectrum antimicrobial capabilities needed to effectively treat the full range of MRSA infections, including pneumonia, bone and joint infections, diabetic foot infections (DFI), and bloodstream infection, highlighting a need for new and improved antibiotic options (4).
About Levonadifloxacin
Levonadifloxacin (IV) and its oral prodrug, alalevonadifloxacin, are novel benzoquinolizine quinolones with broad-spectrum activity against Gram-positive, quinolone-sensitive Gram-negative, atypical, and anaerobic bacteria. They are effective against quinolone-resistant Staphylococcus aureus (QRSA), MRSA, heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA), and respiratory pathogens like S. pneumoniae (penicillin and macrolide-resistant), S. pyogenes, and H. influenzae (5). Both formulations have recently been approved for treating acute bacterial skin and skin structure infections (ABSSSI), including diabetic foot infections (DFI) and concurrent bacteremia (5).
Mechanism of action and unique properties
Levonadifloxacin exerts bactericidal action by inhibiting both DNA gyrase and topoisomerase IV, with a stronger affinity for DNA gyrase, unlike earlier fluoroquinolones such as ciprofloxacin and levofloxacin, which target topoisomerase IV. Levonadifloxacin kills biofilm-embedded QRSA and MRSA and inhibits the NorA efflux pump, which is a main factor in developing quinolone resistance (5).
Unique properties of Levonadifloxacin include:
Broad spectrum coverage against Gram-positive pathogens (including MRSA), respiratory Gram-negative bacteria, atypical and anaerobic bacteria (6).
Highest lung penetration among quinolones (6).
No dose adjustment required for renal or hepatic impairment (6).
Effective against biofilms, especially for MRSA/QRSA biofilms (6).
Excellent cardiovascular and gastrointestinal safety profiles (6).
No QT prolongation, phototoxicity, nephrotoxicity, or hepatotoxicity (7).
Easy transition between intravenous and oral formulations (6).
Efficacy and Safety of Levonadifloxacin
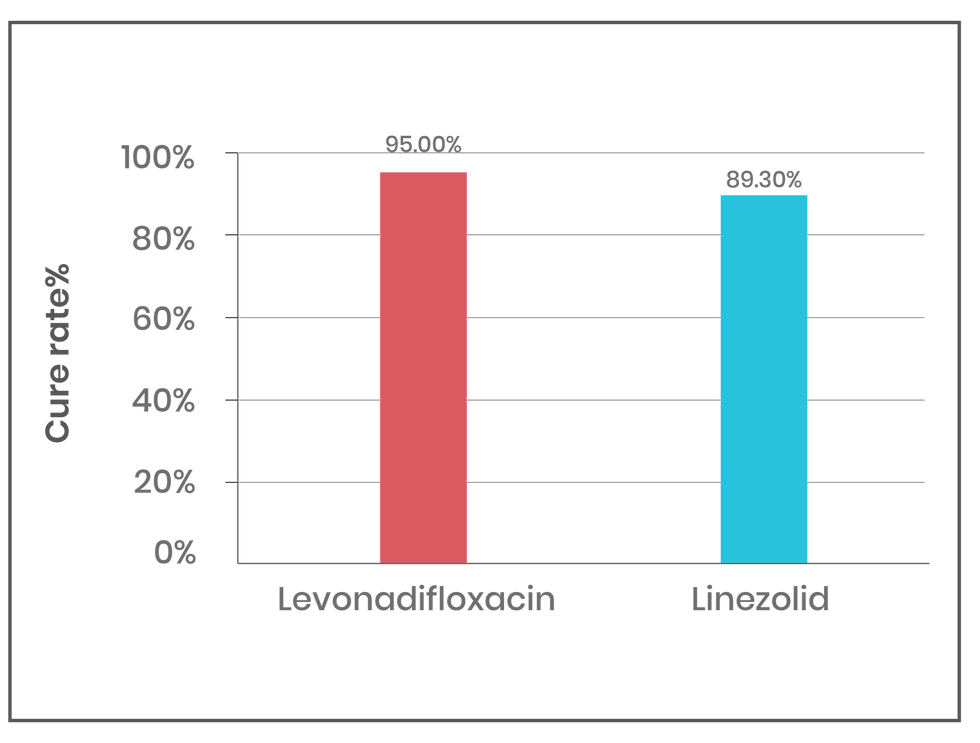
Levonadifloxacin has shown promising efficacy and safety in treating acute bacterial skin and skin structure infections (ABSSSI), community-acquired bacterial pneumonia (CABP), and respiratory infections. In a multicentre Phase 3 trial, levonadifloxacin (1000 mg oral or 800 mg IV) was compared with linezolid (600 mg oral or IV) for treating ABSSSI, including diabetic foot infections. Levonadifloxacin showed a higher clinical response rate than linezolid, with 85.2% for IV and 92.7% for oral formulations at days 3-4. In MRSA patients, levonadifloxacin achieved a 95.0% clinical cure rate compared to 89.3% with linezolid (See figure 1). For diabetic foot ulcers, levonadifloxacin showed a higher cure rate (91.7% vs. 76.9%) (8).
Figure 1: Clinical cure rate in patients with MRSA
A multi-centre, retrospective, post-marketing, real-world descriptive observational study (PIONEER study) was conducted in 338 patients with lower respiratory tract infections (LRTI), including CABP. Investigators found clinical success rates of 93.5% for IV therapy, 98.7% for oral therapy, and 100% for IV followed by oral therapy. Investigators rated levonadifloxacin therapy as "excellent to good" in 95.2% of patients, with a safety rating of "very good" in 97.9%. Only 1.2% of patients experienced minor adverse events, including nausea, diarrhoea, and fatigue (9).
In another prescription event monitoring study (2024) investigators studied the use of oral and/or IV levonadifloxacin over 18 months in 1,266 patients with various bacterial infections, such as skin and soft tissue infections, diabetic foot infections, bone and joint infections, septicaemia, catheter-related bloodstream infections, respiratory infections, febrile and neutropenia, evaluated the use of oral and/or IV levonadifloxacin. The study reported a clinical cure rate of 95.7% by day 8, with a microbial success rate of 93.3%. The study confirmed that levonadifloxacin demonstrates excellent efficacy and safety profile, particularly against resistant pathogens like MRSA and QRSA (10).
Conclusion
As the global challenge of antimicrobial resistance continues to grow, levonadifloxacin offers a promising solution to an unmet medical need, particularly in regions like India, where MRSA prevalence is high and treatment options remain limited.
About the Author
Dr Ujwala Kemkar is a medical writer with a BHMS degree and 10 years of experience in developing medico-marketing material. Her interest lies in creating well-researched, engaging, and impactful content that not only supports healthcare professionals but also educates and empowers patients.
Reference
Salam MA, Al-Amin MY, Salam MT, et al. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare (Basel). 2023;11(13):1946.
Unnikrishnan MK, Akhila Eldo P, Elstin Anburaj S, et al. For whom the bell tolls? Methicillin-resistant Staphylococcus aureus infections in India. J Appl Pharm Sci, 2023; 13(12):015–030.
Reddy PK, Sutar A, Sahu S, et al. Methicillin resistant staphylococcus aureus - importance of appropriate empirical therapy in serious infections. Int J Adv Med. 2022; 9(1):56-65
Veeraraghavan B, Bakthavatchalam YD, Manesh A, et al. India-discovered levonadifloxacin & alalevonadifloxacin: A review on susceptibility testing methods, CLSI quality control and breakpoints along with a brief account of their emerging therapeutic profile as a novel standard-of-care. Indian J Med Microbiol. 2023;41:71-80.
Saseedharan S, Dubey D, Singh RK, Zirpe K, et al. Treatment challenges in the management of difficult-to-treat gram-positive infections: A consensus view apropos therapeutic role of novel anti-MRSA antibiotics, levonadifloxacin (IV) and alalevonadifloxacin (oral). Indian J. Med. Microbiol. 2024; 47: 100528
Mehta Y, Mishra KC, Paliwal Y, et al. Meeting the Unmet Need in the Management of MDR Gram-Positive Infections with Oral Bactericidal Agent Levonadifloxacin. Crit Care Res Pract. 2022;2022:2668199.
Bhagwat SS, Nandanwar M, Kansagara A, et al. Levonadifloxacin, a Novel Broad-Spectrum Anti-MRSA Benzoquinolizine Quinolone Agent: Review of Current Evidence. Drug Des Devel Ther. 2019;13:4351-4365.
Bhatia A, Mastim M, Shah M, et al. Efficacy and Safety of a Novel Broad-Spectrum Anti-MRSA Agent Levonadifloxacin Compared with Linezolid for Acute Bacterial Skin and Skin Structure Infections: A Phase 3, Open label, Randomized Study. J Assoc Physicians India. 2020;68(8):30-36.
Prabhudesai D. P., Jain D. A., Borade D. P., et al. Efficacy and safety of levonadifloxacin in the management of community-acquired bacterial pneumonia (CABP): findings of a retrospective, real-world, multi-centre study. Int. J. Inn. Res. Med Sci. 2021;6(12):919–925.
Saseedharan S, Zirpe K, Mehta Y, et al. Efficacy and Safety of Oral and IV Levonadifloxacin Therapy in Management of Bacterial Infections: Findings of a Prospective, Observational, Multi-center, Post-marketing Surveillance Study. Cureus. 2024;16(2):e55178.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@rxnews.in







Comments