Inclisiran in patients with ASCVD and elevated LDL-C: ORION 11
- Ms. Hima Saxena

- Sep 8, 2025
- 4 min read
Updated: Sep 10, 2025

Atherosclerotic cardiovascular disease (ASCVD) is a progressive condition characterized by plaque build-up within arterial walls, leading to narrowing, stiffening, and ultimately restricted blood flow to vital organs. This process contributes to several major clinical syndromes, including coronary heart disease, ischemic stroke, and peripheral artery disease. Over time, plaque instability or thrombosis may precipitate acute cardiovascular events such as myocardial infarction or transient ischemic attack. In addition to ASCVD itself, risk-equivalent conditions such as type 2 diabetes and chronic kidney disease also confer a comparable risk of cardiovascular morbidity and mortality (1).
Atherosclerotic cardiovascular disease (ASCVD) is the primary contributor to global mortality and disability, responsible for nearly 85% of deaths linked to heart-related conditions. Cases are surging rapidly in India due to a combination of growing elderly populations, widespread risk factors, and insufficient access to quality preventive services (2).
The current standard of care for ASCVD includes high-intensity statin therapy, often combined with ezetimibe, antiplatelet agents, antihypertensives, and lifestyle modifications. While this approach has improved clinical outcomes, many patients continue to experience recurrent events (3). Statin intolerance may also contribute to inadequate LDL cholesterol (LDL-C) control. Additionally, certain patient subgroups, such as those with diabetes or familial hypercholesterolemia often remain undertreated or fail to achieve LDL-C targets (4). These gaps emphasize the need for newer therapeutic modalities that can deliver sustained lipid lowering with improved compliance.
Inclisiran: A Novel Lipid-Lowering Agent
Inclisiran is a novel small interfering RNA (siRNA) therapeutic that reduces LDL-C by targeting hepatic production of proprotein convertase subtilisin/kexin type 9 (PCSK9). Administered via subcutaneous injection, inclisiran is conjugated with N-acetylgalactosamine, which facilitates specific uptake into hepatocytes through asialoglycoprotein receptors. Once inside the liver cell, inclisiran is incorporated into the RNA-induced silencing complex (RISC), where it binds to and degrades PCSK9 messenger RNA. This results in reduced synthesis of PCSK9 protein, which otherwise promotes LDL receptor degradation. By decreasing PCSK9 levels, inclisiran increases the availability of LDL receptors on hepatocytes, thereby enhancing LDL-C clearance from circulation. Notably, inclisiran has a long duration of action, with effective dosing required only twice a year after initial loading doses (5).
Clinical evidence: ORION 11 Study
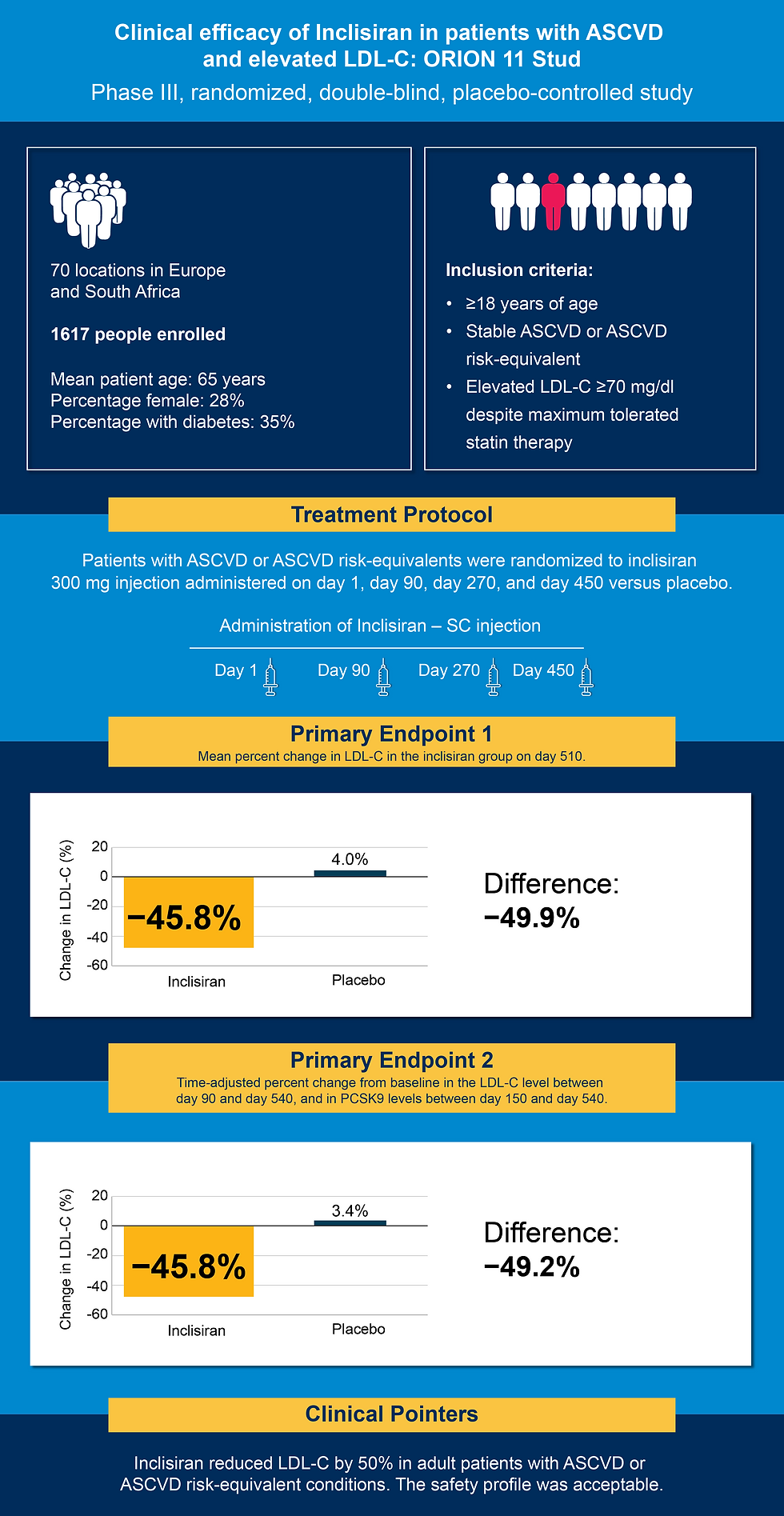
The ORION-11 trial was a pivotal Phase III, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of inclisiran in patients with atherosclerotic cardiovascular disease (ASCVD) or ASCVD risk-equivalent conditions. A total of 1,617 adult patients with elevated low-density lipoprotein cholesterol (LDL-C), despite receiving maximally tolerated statin therapy with or without ezetimibe, were enrolled. Eligible participants had either documented ASCVD or high-risk profiles, including type 2 diabetes, familial hypercholesterolemia, or a calculated 10-year cardiovascular risk of 20% or higher (6).
Participants were randomized 1:1 to receive 300 mg of inclisiran or placebo via subcutaneous injection on day 1, day 90, and every six months thereafter, over a 540-day follow-up period. The percentage change in LDL cholesterol level at day 510 was −45.8% in the inclisiran group and 4.0% in the placebo group resulting in a between-group difference of −49.9% (P<0.001). The corresponding time-adjusted change in LDL cholesterol level was −45.8% with inclisiran and 3.4% with placebo representing a between-group difference of −49.2% (P<0.001) (6).
Inclisiran was well tolerated, with treatment-emergent adverse events occurring in 83% of inclisiran-treated patients and 82% of those on placebo. Serious adverse events were reported in 22% of the inclisiran group and 23% of the placebo group. Mild injection-site reactions were more frequent in the inclisiran arm (4.7% vs. 0.5%). No liver, kidney, or muscle toxicity was observed (6).
Study Summary
Conclusion
Inclisiran demonstrated significant and sustained reductions in LDL-C among patients with ASCVD and those at high cardiovascular risk, with a safety profile comparable to placebo. Its novel siRNA-based mechanism and infrequent dosing schedule offer a practical and effective approach to long-term lipid management. These findings support the use of inclisiran as a valuable therapeutic option in patients who remain inadequately controlled despite standard lipid-lowering therapy.
About the Author
Ms. Hima Saxena is a medical writer and editor with a Master’s in Pharmaceutics and a strong background in medical communications. She creates clear, evidence-based content that supports healthcare professionals and empowers patients. Hima collaborates with pharmaceutical and healthcare clients to deliver accurate, impactful content across diverse therapeutic areas, bridging scientific integrity with accessible communication.
Abbreviations:
ASCVD: Atherosclerotic Cardiovascular Disease; LDL-C: Low-Density Lipoprotein Cholesterol; PCSK9: Proprotein Convertase Subtilisin/Kexin Type 9;
References
Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, et al. Pathophysiology of atherosclerosis. Int J Mol Sci. 2022 Mar 20;23(6):3346.
Cardiovascular diseases (CVDs). Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Accessed May 31, 2025.
Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023 Jul 20;148(9).
Krähenbühl S, Pavik-Mezzour I, von Eckardstein A. Unmet needs in LDL-C lowering: When statins won’t do! Drugs. 2016 Jul 25;76:1175–1190.
Zhang Y, Chen H, Hong L, et al. Inclisiran: a new generation of lipid-lowering siRNA therapeutic. Front Pharmacol. 2023 Oct 13;14:1260921.
Ray KK, Wright RS, Kallend D, Koenig W, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020 Mar 18;382(16):1507–1519.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@crixus.co.in






Comments