Inclisiran in patients with ASCVD and elevated LDL-C: ORION 10
- Ms. Hima Saxena

- Sep 9, 2025
- 4 min read
Updated: Sep 10, 2025
Atherosclerotic cardiovascular disease (ASCVD) is a progressive condition characterized by plaque build-up within arterial walls, leading to narrowing, stiffening, and ultimately restricted blood flow to vital organs. This process contributes to several conditions, including coronary heart disease, ischemic stroke, and peripheral artery disease (1). The disease often shows no symptoms until it reaches advanced stages, highlighting the importance of early intervention and effective lipid management to reduce the risk of future complications (2).
ASCVD is the leading cause of death and disability worldwide, responsible for approximately 85% of cardiovascular deaths. In India and other low- and middle-income countries, the number of cases is increasing rapidly due to aging populations, persistent risk factors, and limited access to optimal preventive care (3).
Management of ASCVD focuses on risk reduction through lifestyle modification and pharmacologic therapy (4). High-intensity statins are the cornerstone for lowering low-density lipoprotein cholesterol (LDL-C), often supplemented with ezetimibe or PCSK9 monoclonal antibodies in high-risk or statin-intolerant patients. Other key therapies include antihypertensives, antiplatelet agents, and occasionally anticoagulants in select populations (5).
Despite these advancements, many patients fail to reach target LDL-C levels or experience recurrent cardiovascular events due to residual risk (4). Additionally, factors like elevated lipoprotein(a) or metabolic syndrome are not fully addressed by current therapies (4). These gaps underscore the need for novel, long-acting therapies with proven lipid-lowering efficacy and favorable adherence profiles.
Inclisiran: A Novel siRNA therapy
Inclisiran is a first-in-class small interfering RNA (siRNA) therapy designed to reduce LDL-C levels by targeting the hepatic production of proprotein convertase subtilisin/kexin type 9 (PCSK9). Once administered subcutaneously, inclisiran binds to asialoglycoprotein receptors on hepatocytes via its N-acetylgalactosamine (GalNAc) conjugate, ensuring liver-specific uptake. Inside the cell, it is incorporated into the RNA-induced silencing complex (RISC), which degrades PCSK9 mRNA and inhibits further protein synthesis (6).
The resulting decrease in circulating PCSK9 levels promotes the recycling of LDL receptors on hepatocytes, enhancing LDL-C clearance. This mechanism allows for sustained LDL-C reduction with dosing only twice a year after an initial loading phase, potentially improving adherence and long-term outcomes (6).
Clinical evidence in ASCVD patients: ORION-10 Study
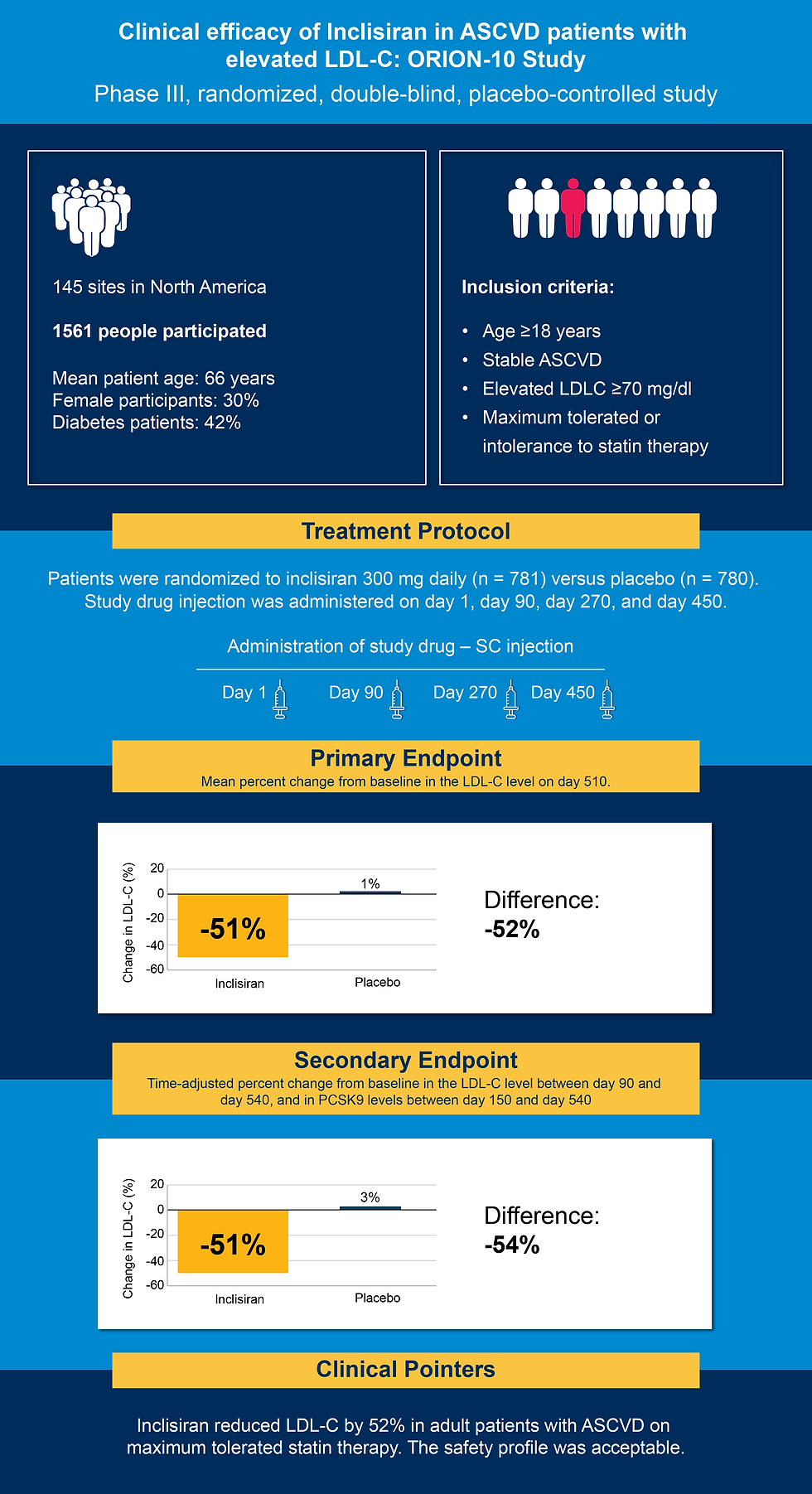
The ORION-10 trial was a Phase III, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of inclisiran in patients with established ASCVD and elevated LDL-C despite receiving maximally tolerated statin therapy. The trial enrolled 1,561 participants (mean age: 66 years; 30% female; 42% with diabetes) across 145 sites in North America. Participants were randomized 1:1 to receive inclisiran 300 mg or placebo, administered subcutaneously on days 1, 90, 270, and 450, with follow-up extending to day 540. All patients had documented ASCVD and baseline LDL-C levels ≥70 mg/dL (7).
Inclisiran achieved a significant and durable reduction in LDL-C levels. At day 510, inclisiran reduced LDL-C levels by 52.3% versus +1% in the placebo group (p < 0.0001(7).
The treatment was well tolerated. The overall incidence of adverse events was comparable between groups (74% inclisiran vs. 75% placebo). Serious adverse events occurred slightly less frequently in the inclisiran group, and no signs of liver, kidney, or muscle toxicity were observed. Injection-site reactions were more common with inclisiran but were generally mild (7).
The ORION-10 demonstrated that inclisiran, when added to standard lipid-lowering therapy, delivers robust, sustained LDL-C reduction in patients with established ASCVD. Its biannual dosing schedule has the potential to address a critical gap in adherence, particularly for patients who struggle with daily or frequent medications. The safety profile of inclisiran was comparable to placebo, with no significant differences in adverse events aside from mild injection-site reactions (7).
Study Summary
Conclusion
The novel mechanism of action of Inclisiran offers an innovative approach to cholesterol management distinct from monoclonal antibodies, yet with comparable efficacy. Given the high global and regional burden of ASCVD and the limitations of existing therapies, inclisiran represents a promising therapeutic advancement.
About the Author
Ms. Hima Saxena is a medical writer and editor with a Master’s in Pharmaceutics and a strong background in medical communications. She creates clear, evidence-based content that supports healthcare professionals and empowers patients. Hima collaborates with pharmaceutical and healthcare clients to deliver accurate, impactful content across diverse therapeutic areas, bridging scientific integrity with accessible communication.
Abbreviations:
LDL-C: Low-Density Lipoprotein Cholesterol; ASCVD: Atherosclerotic Cardiovascular Disease; PCSK9: Proprotein Convertase Subtilisin/Kexin Type 9;
References
Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Rekondo Olaetxea J, Alloza I, Vandenbroeck K, Benito-Vicente A, Martín C. Pathophysiology of atherosclerosis. Int J Mol Sci. 2022 Mar 20;23(6):3346.
Madaudo C, Coppola G, Parlati ALM, Corrado E. Discovering inflammation in atherosclerosis: Insights from pathogenic pathways to clinical practice. Int J Mol Sci. 2024 May 30;25(11):6016.
Cardiovascular diseases (CVDs). Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Accessed May 31, 2025.
Hurwitz M, Agboola OJ, Gami A, Williams MS, Virani SS, Sharma GV, Patel J. Strategies for the secondary prevention of atherosclerotic cardiovascular disease. US Cardiol Rev. 2025 Apr 28;19:e11.
Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, Dixon DL, Williams MS. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023 Jul 20;148(9).
Zhang Y, Chen H, Hong L, Wang H, Li B, Zhang M, Li J, Yang L, Liu F. Inclisiran: a new generation of lipid-lowering siRNA therapeutic. Front Pharmacol. 2023 Oct 13;14:1260921.
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020 Mar 18;382(16):1507–1519.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@rxnews.in






Comments