IRAKLIA Study - Intravenous Isatuximab vs Subcutaneous Isatuximab
- Ms. Roma Joglekar

- Sep 9, 2025
- 2 min read
Updated: Sep 10, 2025
The CD38 are present widely and uniformly on the surface of multiple myeloma cells making it a target for many biological therapeutics. Isatuximab (Sarclisa) is a CD38 monoclonal antibody that binds the CD38 receptor on multiple myeloma cells. Currently, Istuximab can be delivered as a drip through the intravenous route or through the subcutaneous route (1).
About the study
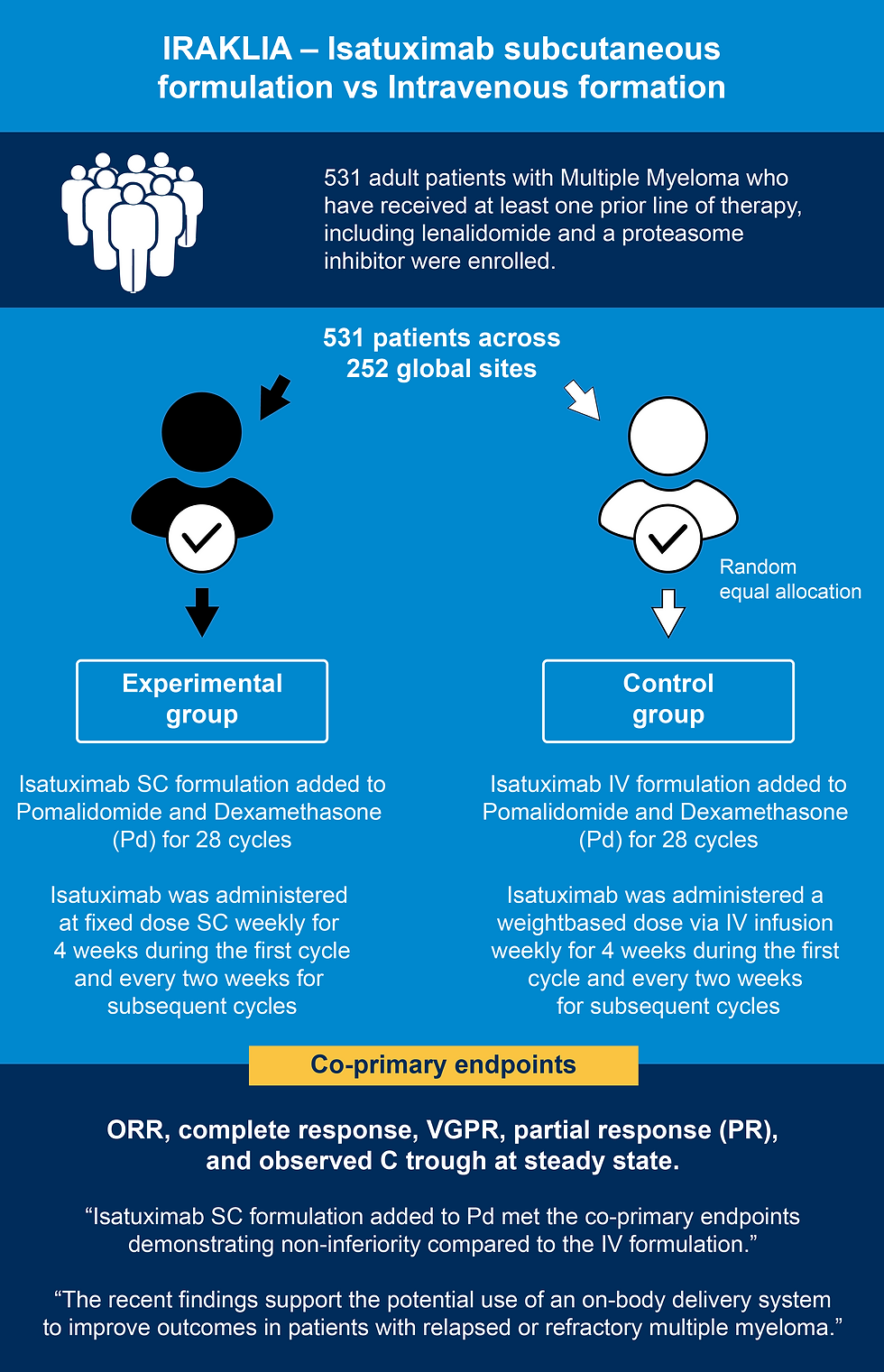
IRAKLIA is a randomized, open-label, pivotal phase 3 study that was conducted to evaluate the non-inferiority of Isatuximab administered at a fixed dose subcutaneously via an OBDS versus weight-based dosed Isatuximab in combination with Pd in adults with Relapsed/Refractory Multiple Myeloma (2).
What is OBDS?
Enable Injections’ enFuse® hands-free on-body delivery system (OBDS), was designed to administer high-volume medicines subcutaneously through an automated drug delivery technology. It is an alternative delivery method designed to improve patient experience (2).
Summary of IRAKLIA Study
About the Author
Ms. Roma Joglekar is a Sports Physiotherapist with three years of on-field experience in helping athletes recover, improve and excel in their performance. Roma is passionate about medical science and has keen interest in simplifying complex medical information and share it with healthcare professionals, patients, care givers and the general population.
Abbreviations
OBDS: On-Body Delivery System; SC: Subcutaneous; IV: Intravenous; RRMM: Relapsed or Refractory Multiple Myeloma; VGPR: very good partial response;
References
A trial looking at different ways of having isatuximab for multiple myeloma that has come back or stopped responding to treatment (IRAKLIA). Available at: https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-looking-at-different-ways-of-having-isatuximab-for-multiple-myeloma-that-has-come-back-or#undefined Accessed on 16 January 2025.
Press Release: New Sarclisa subcutaneous formulation met co-primary endpoints in the IRAKLIA phase 3 study in multiple myeloma. Available at: https://www.sanofi.com/en/media-room/press-releases/2025/2025-01-09-06-00-00-3006798?utm_campaign=sc_2023_copd&utm_source=linkedin_global&utm_medium=social&utm_content=300001535239435 Accessed on 16 January 2025.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@rxnews.in






Comments