LDL-C control with Inclisiran in HeFH patients: ORION 9 study
- Ms. Hima Saxena

- Sep 9, 2025
- 4 min read
Updated: Sep 10, 2025
Heterozygous familial hypercholesterolemia (HeFH) is a prevalent genetic disorder marked by elevated low-density lipoprotein cholesterol (LDL-C) levels from birth, often due to mutations in the LDLR gene (1). This leads to impaired clearance of LDL-C from the bloodstream, significantly increasing the risk of early-onset atherosclerotic cardiovascular disease (ASCVD), including myocardial infarction and stroke (2). Without timely diagnosis and intervention, individuals with HeFH are prone to cardiovascular events decades earlier than the general population (3).
Globally, HeFH affects approximately 1 in 250 individuals, equating to an estimated 30 million people. In India alone, this could translate to around 5 million affected individuals, although actual numbers may be underestimated due to underdiagnosis and limited awareness (4).
HeFH management traditionally involves a combination of lifestyle modifications and pharmacotherapy—primarily statins, often supplemented with ezetimibe. While these approaches help lower LDL-C, many patients fail to reach recommended LDL targets even on maximally tolerated therapy. Additionally, adherence can be an issue due to daily pill burden and side effects. There remains an unmet need for therapies that provide sustained efficacy with simplified dosing regimens and fewer side effects (5).
Inclisiran: A Novel siRNA Therapy
Inclisiran represents a novel class of LDL-C lowering treatments, utilizing small interfering RNA (siRNA) to reduce PCSK9 levels. It is conjugated with N-acetylgalactosamine (GalNAc) to enable targeted delivery to hepatocytes, the primary site of cholesterol metabolism (6).
After subcutaneous administration, inclisiran binds to asialoglycoprotein receptors on liver cells and is incorporated into the RNA-induced silencing complex (RISC). The RISC uses inclisiran to degrade PCSK9 mRNA, preventing PCSK9 protein synthesis. Reduced PCSK9 levels promote LDL receptor recycling and increase receptor density on hepatocyte surfaces, thereby enhancing LDL-C clearance from the circulation. This mechanism enables effective LDL-C reduction with only a few doses annually, offering a promising alternative for long-term lipid management in HeFH (6).
Clinical Evidence: ORION-9 Study.
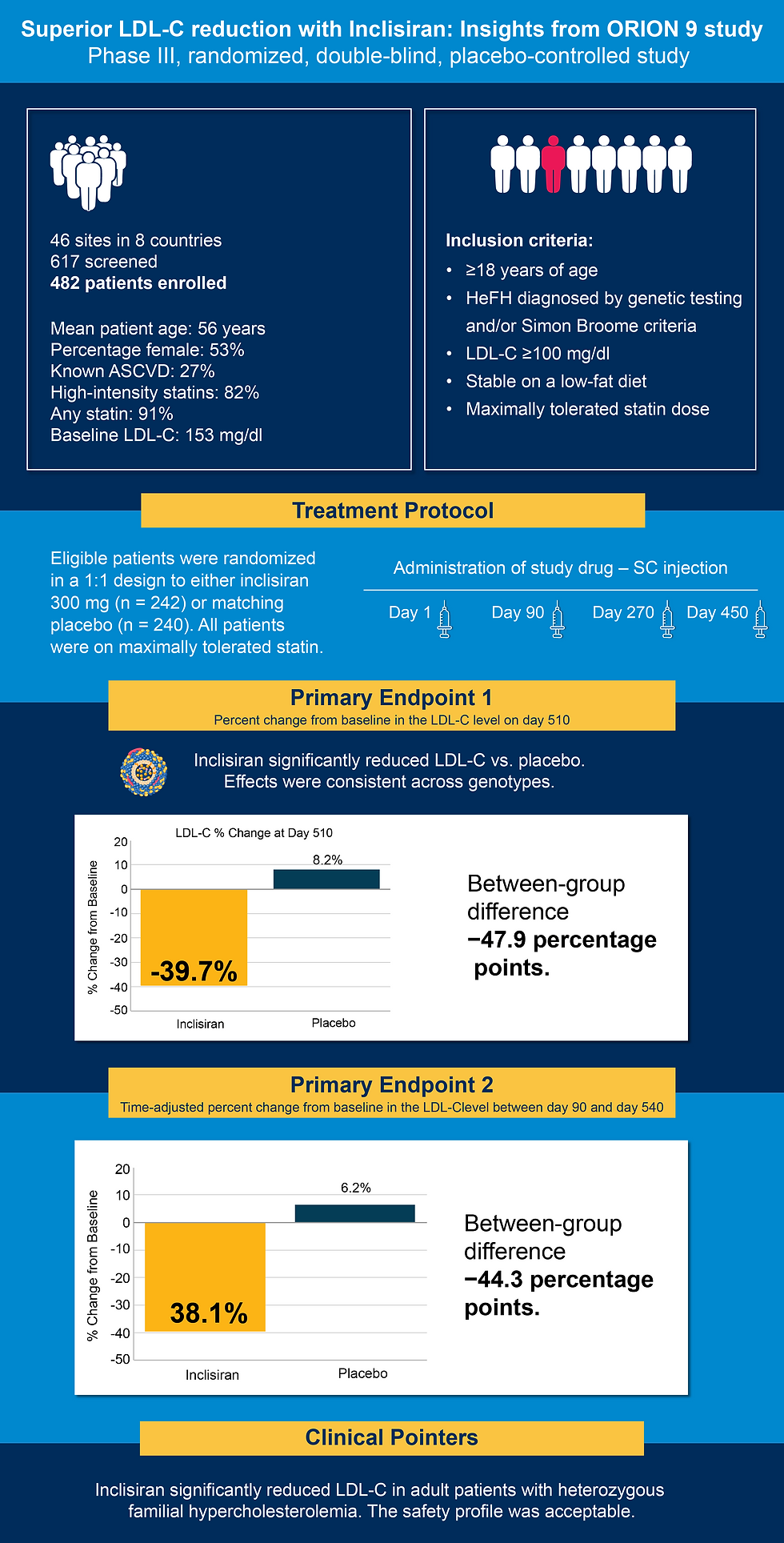
The ORION-9 trial was a Phase III, randomized, double-blind, placebo-controlled study assessing inclisiran in adults with HeFH. Conducted across 46 sites in eight countries, the trial enrolled 482 patients with genetically or clinically confirmed HeFH who had elevated LDL-C levels (≥100 mg/dL) despite being on maximally tolerated statin therapy, with or without ezetimibe (1).
Participants were randomized 1:1 to receive either inclisiran 300 mg or placebo. Injections were administered subcutaneously on days 1, 90, 270, and 450. Patients were followed for 510 days. The cohort had a mean age of 56 years, and 53% were female. Most were on high-intensity statins (82%) and had a baseline LDL-C of 153 mg/dL (1).
Inclisiran significantly reduced LDL-C levels compared to placebo. At day 510, the mean percent LDL-C reduction from baseline was –39.7% with inclisiran versus +8.2% with placebo, amounting to a between-group difference of 47.9 percentage points (p < 0.0001). The time-adjusted LDL-C reduction from days 90 to 540 was –44.3 percentage points for inclisiran versus +6.2% for placebo (1).
Additionally, 65% of inclisiran-treated patients achieved LDL-C levels below 100 mg/dL, despite already being on statin therapy. These effects were consistent across patients with various monogenic LDLR mutations. Inclisiran was generally well tolerated. The most common adverse events included nasopharyngitis and injection-site reactions. Rates of transaminitis and all-cause mortality were low and similar between groups. No new safety signals emerged over the 16-month trial period (1).
Study Summary
Conclusion
The ORION-9 study establishes inclisiran as a promising treatment approach for familial hypercholesterolemia (HeFH), particularly in patients not adequately controlled with statins. Its twice-yearly dosing and liver-targeted RNA interference mechanism offer a convenient and sustained method to lower LDL-C. Additionally, inclisiran reduces lipoprotein(a), a cardiovascular risk factor not addressed by statins, which may contribute to further risk reduction. Inclisiran offers a treatment approach for HeFH characterized by sustained efficacy, an acceptable safety profile, and convenient dosing.
About the Author
Ms. Hima Saxena is a medical writer and editor with a Master’s in Pharmaceutics and a strong background in medical communications. She creates clear, evidence-based content that supports healthcare professionals and empowers patients. Hima collaborates with pharmaceutical and healthcare clients to deliver accurate, impactful content across diverse therapeutic areas, bridging scientific integrity with accessible communication.
Abbreviations:
LDL-C: Low-Density Lipoprotein Cholesterol; HeFH: Heterozygous Familial Hypercholesterolemia.
References
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020 Mar 18;382(16):1520–1530.
Kataoka Y, Funabashi S, Doi T, et al. How can we identify very high-risk heterozygous familial hypercholesterolemia? J Atheroscler Thromb. 2022 Jan 13;29(6):795–807.
Mytilinaiou M, Kyrou I, Khan M, et al. Familial hypercholesterolemia: New horizons for diagnosis and effective management. Front Pharmacol. 2018 Jul 12;9:707.
Sawhney JPS, Prasad SR, Sharma M, et al. Prevalence of familial hypercholesterolemia in premature coronary artery disease patients admitted to a tertiary care hospital in North India. Indian Heart J. 2019 Jan 3;71(2):118–122.
Lambert CT, Sandesara P, Isiadinso I, et al. Current treatment of familial hypercholesterolaemia. Eur Cardiol. 2014 Dec;9(2):76–81.
Zhang Y, Chen H, Hong L, et al. Inclisiran: A new generation of lipid-lowering siRNA therapeutic. Front Pharmacol. 2023 Oct 13;14:1260921.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@rxnews.in






Comments