Sacubitril/Valsartan improved Quality of Life in HFrEF patients: PROVIDE HF Study
- Dr. Sheetanshu Grover

- Sep 9, 2025
- 4 min read
Updated: Sep 10, 2025

Heart failure (HF) is a clinical condition characterised by structural or functional abnormality that impairs ventricular filling or ejection of blood to meet the body’s requirement (1,2). Prevalence of HF is accelerating with an estimated increase of 34% in the last few decades, further raising the burden on global healthcare systems (3,4). HF is one of the leading causes of mortality, morbidity, and poor health-related quality of life (QoL) resulting from decreased exercise capability of patients (1,2,5). Introduction of Sacubitril/valsartan has significantly improved the treatment outcomes in patients with HF (2, 5).
Sacubitril/valsartan (ENTRESTO) is the first-in-class angiotensin receptor-neprilysin inhibitor (ARNI). The simultaneous blocking of renin-angiotensin-aldosterone system and inhibition of neprilysin by sacubitril/valsartan increases the levels of natriuretic peptides, which promote vasodilation, natriuresis, and diuresis. This synergistic effect of valsartan and sacubitril provides a more comprehensive approach to manage the complex pathophysiology of HF compared to traditional angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) alone (6).
Sacubitril/valsartan is primarily indicated for patients with chronic heart failure with reduced ejection fraction (HFrEF), specifically those classified as NYHA class II-IV (5). Clinical trials have demonstrated significant reductions in all-cause and cardiovascular mortality, and HF hospitalization in this population compared to ACE inhibitors/ARB (2,7). Improvement in mortality and morbidly is not always accompanied by better QoL (5).
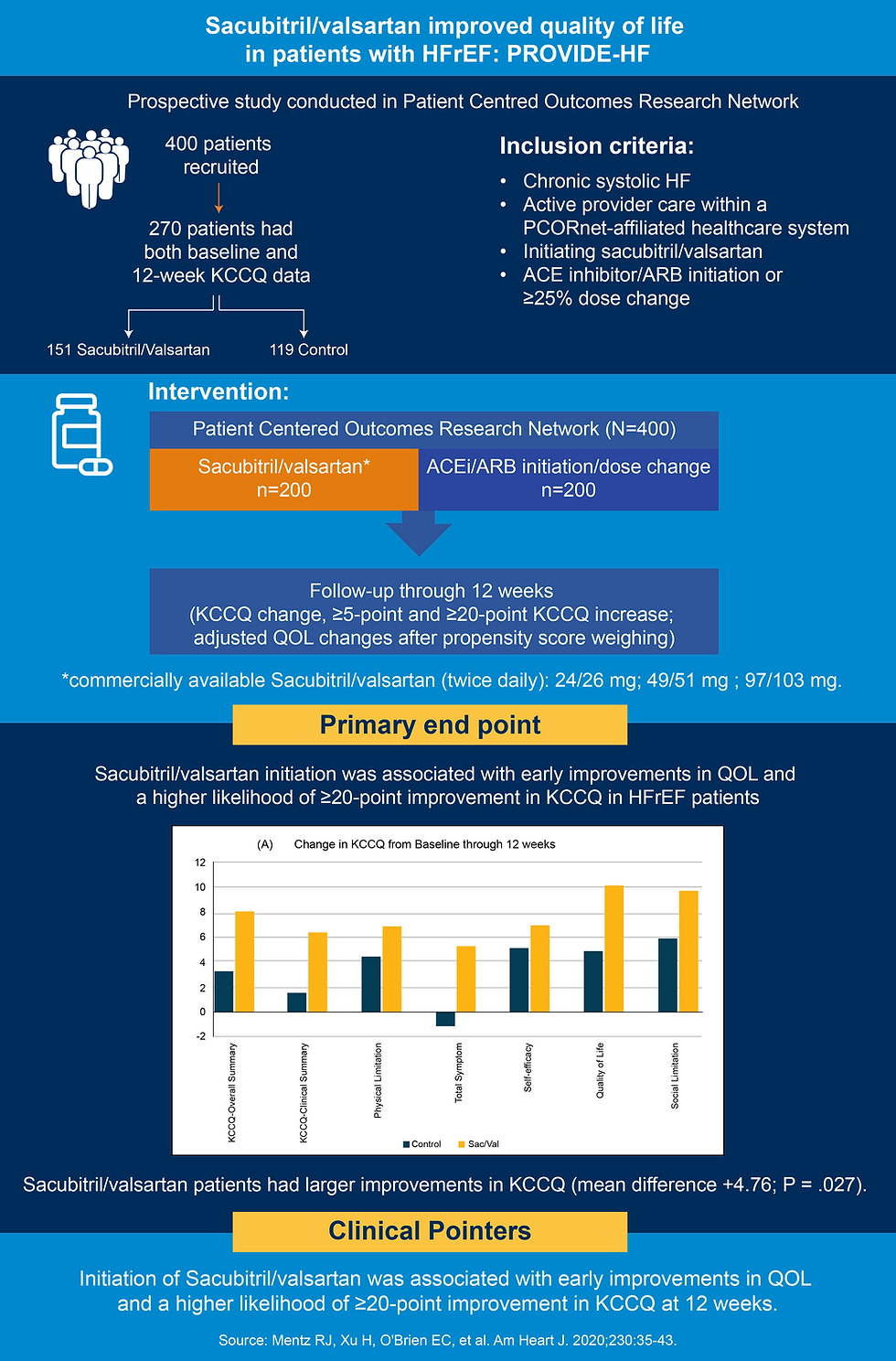
PROVIDE-HF, a prospective study compared sacubitril/valsartan versus ACE inhibitor/ARB initiation. This study, conducted in Patient Cantered Outcomes Research Network provides real-world evidence for improvements in QoL scores with sacubitril/valsartan (8).
Summary of the study
Guidelines and Recommendations
ACC/AHA HF Consensus 2024 recommends sacubitril/valsartan for patients with HFrEF and NYHA class II to IV symptoms to reduce morbidity and mortality (4,9). Patients can be with or without prior ACE inhibitors/ARBs treatment. Individuals transitioning from an ACEi to sacubitril/valsartan should undergo a 36-hour washout period. The Guideline recommends the initial dose of 24/26 mg twice a day (bid) if systolic blood pressure (SBP) is >100 mmHg. The dose can be gradually increased to 97/103 mg bid after 3-4 weeks if no improvement is seen. Patients are advised to follow-up after 1 week and then 2 weeks, after discharged. Medication must be discontinued if eGFR is <15 mL/min/1.73 m2 and serum potassium >5.2 mEq/L (4).
Conclusion
In conclusion, sacubitril/valsartan represents a significant advancement in the management of heart failure. Its unique mechanism of action, coupled with robust clinical trial data, makes it a valuable option for patients with HFrEF. By understanding its indications, contraindications, and practical considerations, sacubitril/valsartan can be effectively integrated into our clinical practice and improve patient outcomes.
Dosage
Sacubitril/valsartan is available as film-coated tablets in three strengths: 24/26 mg, 49/51 mg and 97/103 mg, prescribed to be taken orally twice a day (bid) (10).
About the Author
Dr. Sheetanshu Grover is a Medical Writer with a decade of experience in sub-editing, data verification, and proofreading pharmaceutical content. She has refined her expertise of writing in medico-marketing communications while working within Agile POD environments. With a strong background in team leadership and client management, she brings both strategic insight and editorial precision to her work. Dr. Grover has authored four publications based on her master's and doctoral research. She recently launched a blog, phDMaa.wordpress, where she shares few insights from science alongside her full-time role.
Abbreviations
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CI: confidence interval; HFrEF: heart failure with reduced ejection fraction; KCCQ: Kansas City Cardiomyopathy Questionnaire.
References
Zhang R, Sun X, Li Y, He W, et al. The Efficacy and Safety of Sacubitril/Valsartan in Heart Failure Patients: A Review. J Cardiovasc Pharmacol Ther. 2022 Jan 1;27:10742484211058681.
Song Y, Zhao Z, Zhang J, et al. Effects of sacubitril/valsartan on life quality in chronic heart failure: A systematic review and meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2022 Aug 3;9:922721.
Abdin A, Schulz M, Riemer U, et al. Sacubitril/valsartan in heart failure: efficacy and safety in and outside clinical trials. ESC Heart Fail. 2022 Dec;9(6):3737–50.
Maddox, T, Januzzi, J, Allen, L. et al. 2024 ACC Expert Consensus Decision Pathway for Treatment of Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. JACC. 2024 Apr, 83 (15) 1444–1488.
Rubio Campal JM, Del Castillo H, Arroyo Rivera B, et al. Improvement in quality of life with sacubitril/ /valsartan in cardiac resynchronization non-responders: The RESINA (RESynchronization plus an Inhibitor of Neprilysin/Angiotensin) registry. Cardiol J. 2021 May 25;28(3):402–10.
Nicolas D, Kerndt CC, Patel P, et al. Sacubitril-Valsartan. [Updated 2024 Feb 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507904/
Giovinazzo S, Carmisciano L, Toma M, et al. Sacubitril/valsartan in real‐life European patients with heart failure and reduced ejection fraction: a systematic review and meta‐analysis. ESC Heart Fail. 2021 Oct;8(5):3547–56.
Mentz RJ, Xu H, O’Brien EC, et al. PROVIDE-HF primary results: Patient-Reported Outcomes inVestigation following Initiation of Drug therapy with Entresto (sacubitril/valsartan) in heart failure. Am Heart J. 2020 Dec;230:35-43.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation [Internet]. 2022 May 3 [cited 2025 Apr 29];145(18). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
Prescribing Information. Available at: https://www.ema.europa.eu/en/documents/product-information/entresto-epar-product-information_en.pdf Accessed on 16 May, 2025.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@rxnews.in






Comments