Sacubitril/Valsartan showed a greater reduction of NT-proBNP in HFpEF patients: PARAMOUNT Study
- Dr. Anuradha Mallya

- Sep 9, 2025
- 3 min read
Updated: Sep 10, 2025

Patients with documented left ventricular ejection fraction (LVEF) ≥ 50% are considered to have heart failure (HF) with preserved ejection fraction (HFpEF) (1). While HFpEF accounts for ~ 50% of patients with HF globally, it accounts for 15%–20% of patients with HF in India (2).
HFpEF is characterized by increased left ventricular filling pressures and/or reduced cardiac output either at rest or on exertion. The body maintains the cardiac output by abnormally increasing the filling pressure, which manifests as the signs and symptoms of HFpEF (3).
Sodium-glucose co-transporter-2 inhibitors (SGLT2is) are established as mainstay therapy for HFpEF, demonstrating significant benefits, particularly in reducing hospitalizations in both diabetic and nondiabetic populations (2).
Angiotensin receptor neprilysin inhibitors (ARNIs; sacubitril-valsartan) are efficient in select patients with HFpEF,2 offering the following benefits (4):
Reduce the risk of mortality and hospitalization
Reverse cardiac remodeling
Regulate biomarkers of HF
Significantly improve quality of life
Improve renal dysfunction
Provide a better safety profile when compared with angiotensin-converting enzyme inhibitors (ACEIs)/ angiotensin II receptor blockers (ARBs)
About PARAMOUNT study
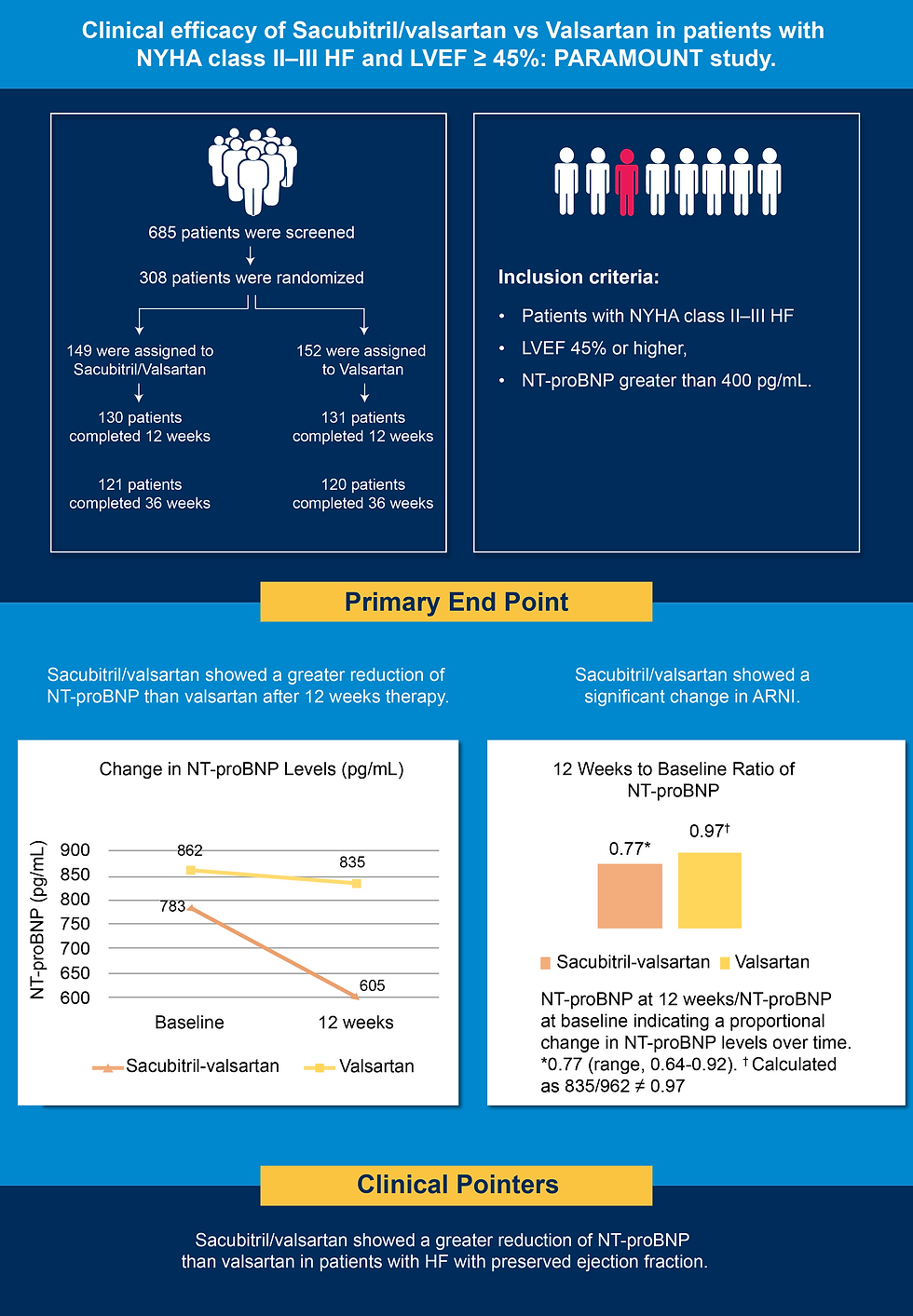
The clinical efficacy of sacubitril-valsartan has been established in several landmark trials. Chopra et al conducted a phase 2, randomized, parallel-group, double-blind, multicenter PARAMOUNT study on 308 patients to assess the efficacy and safety of sacubitril/valsartan vs valsartan alone in patients with chronic HFpEF (5).
Summary of the study
About the Author
Dr. Anuradha Mallya, is a periodontist and a BELS-certified medical editor with a keen interest in making medical science more accessible. With over 5 years of experience in editing clinical and scientific content for global clients, she is passionate about simplifying complex medical information and presenting it in a way that is clear, accurate, and valuable to patients, caregivers, and healthcare professionals alike.
References
Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):352-380.
Harikrishnan S, Oomman A, Jadhav UM, et al. Heart failure with preserved ejection fraction: management guidelines (from Heart Failure Association of India, endorsed by Association of Physicians of India). J Assoc Physicians India. 2022;70(8):71-73.
Vergaro G, Aimo A, Prontera C, et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int J Cardiol. 2019;296:91-97.
Zhang R, Sun X, Li Y, et al. The efficacy and safety of sacubitril/valsartan in heart failure patients: a review. J Cardiovasc Pharmacol Ther. 2022;27:10742484211058681.
Chopra HK, Wander GS, Nair T, et al. Angiotensin receptor-neprilysin inhibitor therapy and cardiac remodeling in heart failure: Consensus Statement from India. J Assoc Physicians India. 2023;71(4):11-12.
Disclaimer
The matter published on this platform has been developed by independent medical writers from various healthcare backgrounds including members of MedWriters Alumni Network. Although great care has been taken in compiling and checking the information, the authors, Rxnews team and its partners or agents, and sponsors shall not be responsible or in any way liable for any errors, omissions, or inaccuracies in this blog article whether arising from negligence or otherwise, however or for any consequences arising therefrom. The inclusion and exclusion of any product do not mention that the publisher advocates or rejects its use generally or in any particular field or field. For any complaints or feedback please write to content@rxnews.in






Comments